About Us
Guangzhou World Medical Devices Co., Ltd. was formerly known as South China Liangjian Technology and Trade of Guangzhou Shuhan Medical. The company's English name, Swordental Co., LTD, has been used up to now, and its registered trademarks are SWORDENTAL and WOLF’S FANG.

Focusing on the production and manufacturing of dental diamond needles for 20 years
For two decades, the company has been dedicated to the manufacturing of dental diamond burs. Subsequently, it has successively developed and produced a variety of dental products, including tungsten carbide burs, endodontic files, delivery needles, broaches, PG drills, polishing and grinding heads, dental brushes, irrigation needles, absorbent points, and gutta-percha points. Our products are renowned globally for their stable quality, diverse models, and reasonable prices.
Qualifications and Certificates
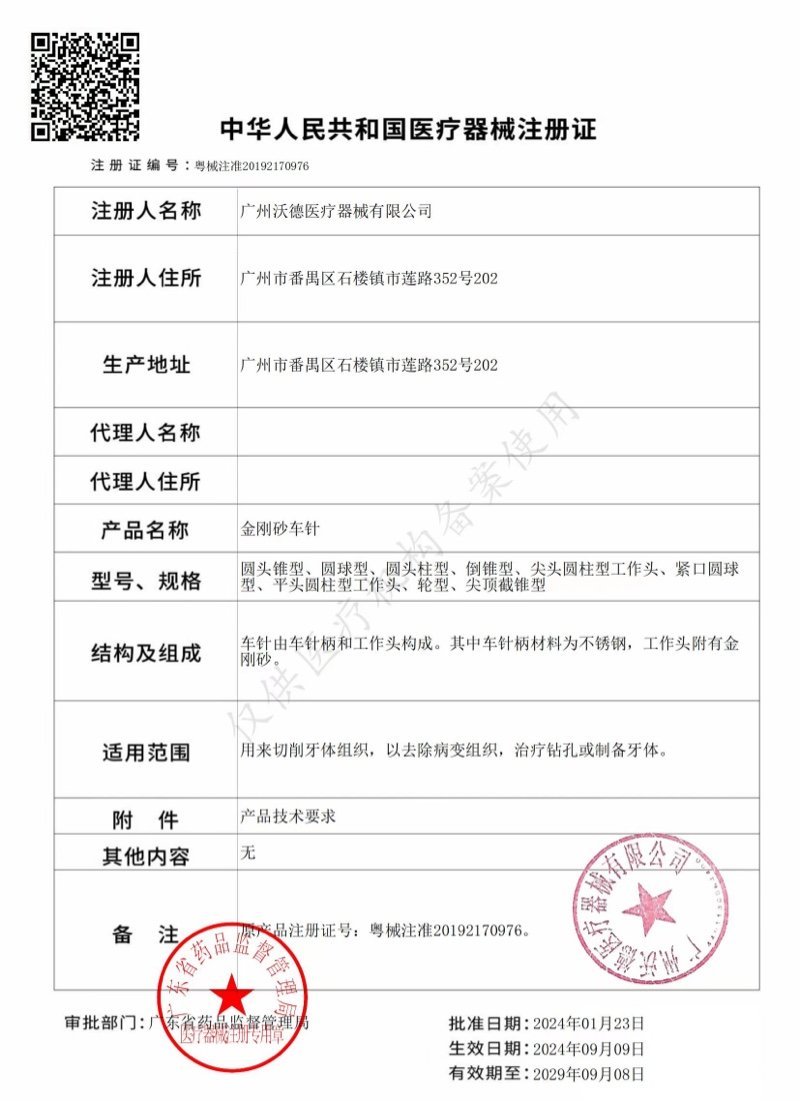
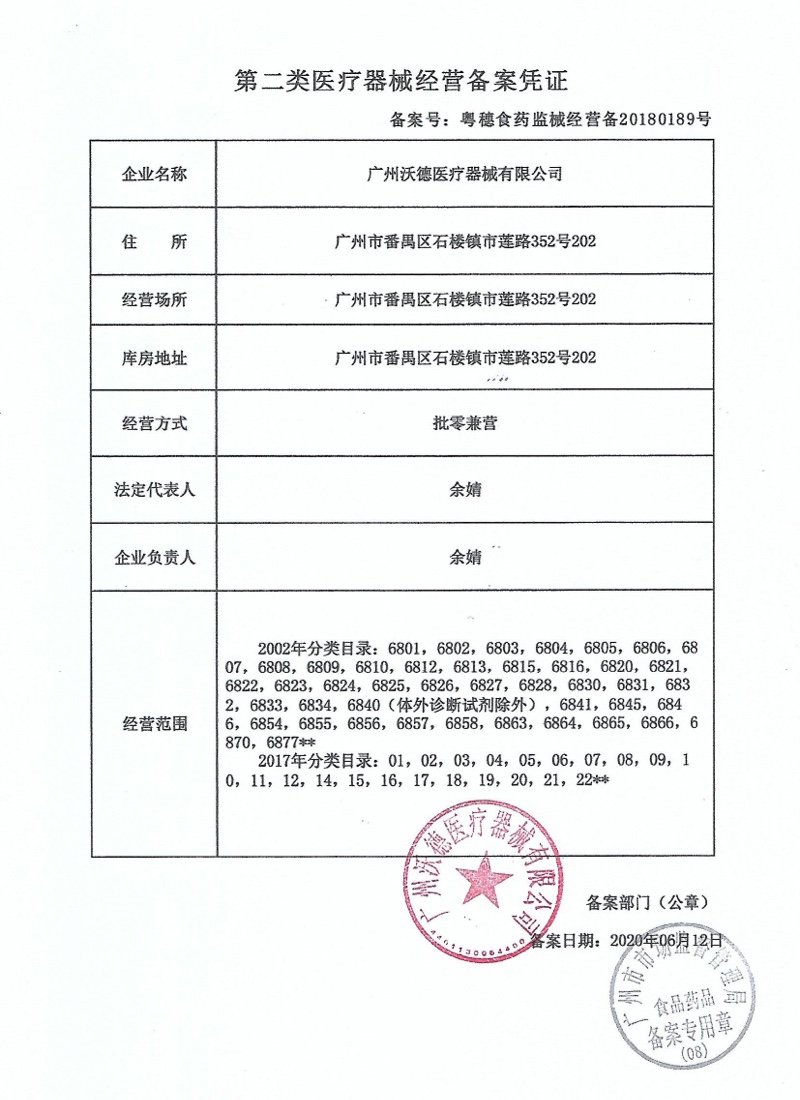
All product lines of the company are registered in China. They have obtained registration certificates or filing certificates issued by the China Food and Drug Administration (CFDA). Meanwhile, the company holds an ISO13485 certificate, a Free Sale Certificate, and an Export Sales Record.
Main Product - Diamond Burs
Excellent Performance: They are sharp and wear - resistant.
As the company's flagship product, diamond burs have a production history of 20 years and are commonly used dental consumables for tooth grinding in stomatology.

Product and Factory Advantages
1
High - quality Materials
Double - layer sand - plating, using a mixture of natural and synthetic diamonds for processing, ensuring product quality and performance.
2
Precision Technology
The blank is processed by a precision CNC lathe. After demagnetization, rounding, and heat treatment, both the handle and the working head meet ISO standards, with high concentricity, causing no damage to dental equipment.
3
Complete Range of Models
There are currently 850 in - stock models and 1000 models in the database. Customization is also available.
4
Stable Quality
The mature production process ensures consistent quality across batches, with the defect rate controlled within 0.05%.